Oli Dugmore 4am - 7am
1 May 2020, 21:25
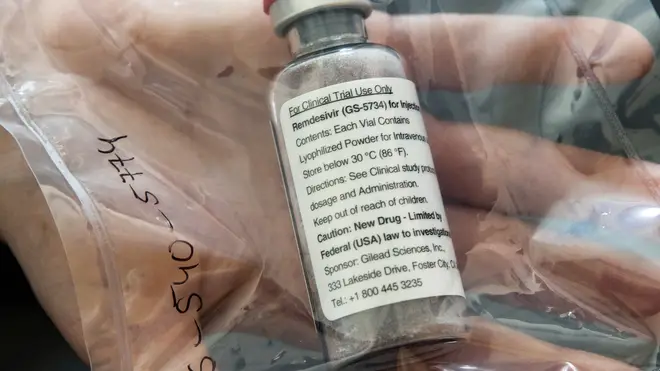
US regulators have allowed emergency use of an experimental drug that appears to help some coronavirus patients recover faster.
The Food and Drug Administration (FDA) acted after preliminary results showed that remdesivir shortened the time to recovery by 31 per cent, or about four days on average, for hospitalised Covid-19 patients.
The study of 1,063 patients is the largest and most strict test of the drug and included a comparison group that received just usual care so remdesivir's effects could be rigorously evaluated.
It is the first drug shown to help fight Covid-19, which has killed more than 230,000 people worldwide.
President Donald Trump announced the FDA's action at the White House.
Those given the drug were able to leave the hospital in 11 days on average versus 15 days for the comparison group.
Listen & subscribe: Global Player | Apple Podcasts | Google Podcasts | Spotify
The drug also might be reducing deaths, although that is not certain from the partial results revealed so far.
The National Institutes of Health's Dr Anthony Fauci said the drug would become a new standard of care for severely ill Covid-19 patients like those in this study.
The drug has not been tested on people with milder illness, and currently is given through an IV in a hospital.
Gilead Sciences has said it would donate its currently available stock of the drug and is ramping up production to make more.
The FDA said the intravenous drug would be specifically for hospitalised patients with "severe disease," such as those experiencing breathing problems requiring supplemental oxygen or ventilators.
FDA commissioner Stephen Hahn said "this was lightning speed in terms of getting something approved", calling the drug "an important clinical advance".
Dr Sameer Khanijo, a critical care specialist, said he wants to see additional studies to clarify the drug's benefit.
"I don't think this is a cure yet, but I think it's starting to point us in the right direction," said Dr Khanijo of North Shore University Hospital in New York. "As a society it's nice to have something that will help stem the tide of this disease."
The FDA said preliminary results for federal researchers warranted Friday's decision, though regulators acknowledged "there is limited information known about the safety and effectiveness of using remdesivir".
The drug's side effects include potential inflammation of the liver and problems related to its infusion, which could lead to nausea, vomiting, sweating and low blood pressure. Information about dosing and potential safety issues will be provided to doctors and patients, the FDA said.
The drug, which blocks an enzyme the virus uses to copy its genetic material, has not been tested on people with milder illness.
The FDA authorised the drug under its emergency powers to quickly speed experimental drugs, tests and other medical products to patients during public health crises.
In normal times the FDA requires "substantial evidence" of a drug's safety and effectiveness, usually through one or more large, rigorously controlled patient studies.
But during public health emergencies the agency can waive those standards and require only that an experimental drug's potential benefits outweigh its risks.
No drugs are approved now for treating the coronavirus, and remdesivir will still need formal approval.
The FDA can convert the drug's status to full approval if Gilead or other researchers provide additional data of remdesivir's safety and effectiveness.
"This is a very, very early stage so you wouldn't expect to have any sort of full approval at this point," said Cathy Burgess, an attorney specialising in FDA issues. "But obviously they want to get this out to patients as quickly as possible."